A very interesting study confirming previous reports on the possibility of using low doses of bevacizumab. A dose of 1 mg / kg / 2 weeks looks very strange. However, to the surprise, the authors report a similar effect with other doses and less toxicity! Your opinion?
METHODS:
From September 2013 to August 2016, we recruited patients with progressive glioblastoma, whatever the previous treatments. We compared a routine control group (CG) of ten mg/kg, to a low dose group (LDG) composed of 5 subgroups: G5: five mg/kg, G4: four mg/kg, G3: three mg/kg, G2: two mg/kg, G1: one mg/kg; each patient was treated with the same dose every two weeks.
RESULTS:
Fifty-three patients were treated: 20 women and 33 men, 24 in the CG and 29 in the LDG. The median age at diagnosis was 62 years [35.0-77.0]. No statistical difference was found in overall survival either for the CG or the LDG (P=0.086) or among groups (P=0.251), with even a trend toward improvement for LDG: 62 weeks [20-145] versus 73 weeks [18-178]. The median progression free survival was comparable: 19.5 weeks [6.0-54.0] for the CG and 15.0 weeks [0.0-134.0] for the LDG (P=0.221). Bevacizumab was stopped either due to progression (45.1%) or toxicity (52.9%), without significant differences between doses but maybe less toxicities in the LDG (16.7% for toxicity in G1).
DISCUSSION:
Use of bevacizumab at progression at lower than usual doses seems to give the same results as the standard dose without giving additional toxicity.

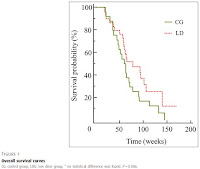

It's difficult to comment without access to the full paper. There have been several retrospective reports suggesting equivalent efficacy of a half-dose of Avastin (5 mg/kg every 2 weeks), with fewer side effects but until now there's been no prospective trial data.
ReplyDeleteMost randomized phase 2 trials are larger than this, with upwards of 40 patients in each arm (this trial had only 24 and 29 patients in the control and low dose groups). The smaller the numbers of patients, the larger the effect size has to be to show statistical significance. And the larger number of patients, the smaller the effect size needs to be to show statistical significance.
However this is a step forward from previous studies in that it sounds like it was a randomized prospective clinical trial. It will be interesting to see the full data.
We should note that Bevacizumab in no dose (even the 10 mg/kg) has affected the overall survival, which this study uses as read-out. So the data are in fact not surprising at all.
ReplyDeleteI believe that many of the placebo arm patients crossing over to receive BEV at recurrence obscured some of the overall survival benefit in the two large phase 3 trials (Avaglio and RTOG0825). There is some data showing that BEV does extend overall survival when given upfront *for patients who don't receive second line therapy*.
Deletehttps://www.ncbi.nlm.nih.gov/pubmed/27006178
Upfront bevacizumab may extend survival for glioblastoma patients who do not receive second-line therapy: an exploratory analysis of AVAglio.
However, unless BEV is needed to treat edema in the newly diagnosed setting I feel its best to save BEV for recurrence rather than upfront, unless its likely the patient won't be well enough to receive second-line treatment.
What do you think about the dose of Avastin 1mg/kg/2 weeks? The authors of the study report the same effect as at other doses and significantly lower toxicity. We want to try this dose from the next Avastin cycle, but it looks very small.
ReplyDeleteThere were only 29 patients in all of the low dose groups combined. If these patients were distrubted equally into all five low dose subgroups, that means there were only 5 to 6 patients in each low dose subgroup. That's not enough patients to properly compare between each of the low dose subgroups, with any kind of significance.
Delete